On August 19, 2014, the Annals of Internal Medicine published a paper titled Atypical Antipsychotic Drugs and the Risk for Acute Kidney Injury [AKI] and Other Adverse Outcomes in Older Adults. The authors were Joseph Hwang et al, and the study was conducted at the Institute for Clinical Evaluative Sciences in Ontario, Canada. The primary funding source was the Academic Medical Organization of Southwestern Ontario. The principal investigator was Amit X. Garg, MD, PhD, a kidney specialist at the London Health Science Center and the London Kidney Clinical Research Unit in Ontario, Canada.
Here is the authors’ conclusion:
“Atypical antipsychotic drug use is associated with an increased risk for AKI and other adverse outcomes that may explain the observed association with AKI. The findings support current safety concerns about the use of these drugs in older adults.”
CONTEXT
By way of background, the authors point out that each year “…millions of older adults worldwide are prescribed atypical antipsychotic drugs (quetiapine, risperidone, and olanzapine),” and that these drugs are “…frequently used to manage behavioral symptoms of dementia, which is not an approved indication…”
The research is based on a retrospective matched pair cohort study using information drawn from healthcare databases.
METHOD
From the databases, the researchers identified 215,543 Ontario residents who received a new oral antipsychotic prescription for an atypical neuroleptic (quetiapine, risperidone, or olanzapine) between June 2007 and December 2011. For the same time period they identified 1,726,930 residents who did not receive a new prescription for any of these products.
From these populations, they identified 97,777 neuroleptic drug recipients, and the same number of drug non-recipients, matched on eleven health status factors. They then checked the matching against 91 measured characteristics. The authors report that: “The 2 groups were well-balanced and showed no meaningful differences in the 91 measured baseline characteristics…” The percentages for the two groups on the 91 characteristics are set out in the report, and the groups are indeed extremely well matched.
RESULTS
The study’s results are presented in Table 1.
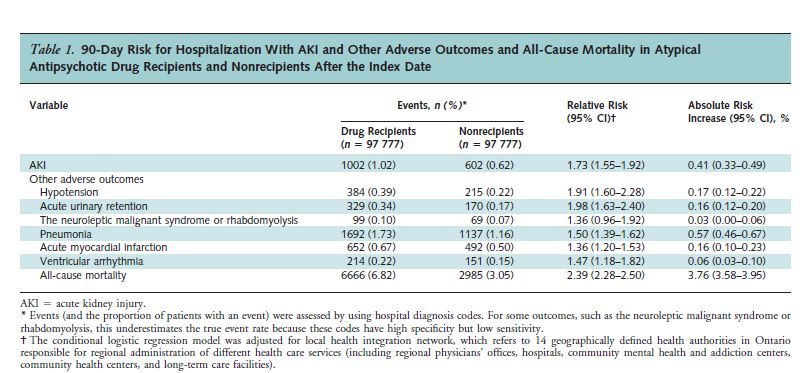
As can be seen, the risk of acute kidney injury was 1.73 times greater for the drug recipients than for the non-recipients. Also, note that the risk for all-cause mortality was 2.39 times greater for the drug recipients.
There were 3681 “excess” deaths from all causes in the drug group during the 90-day period. That amounts to 3764 per 100,000 – almost 4%!
Because this was an observational study, as opposed to a randomized, controlled trial, it is not possible to say definitively that the drugs caused the excess kidney failures and deaths. However, the authors did take great pains to identify and eliminate confounding factors. For instance, they repeated the analysis for the 180 days preceding the index date, and found no difference in the incidence of AKI in the drug vs. non-drug groups prior to the administration of the neuroleptic drug. (relative risk 1.02).
The authors also point out that the excess mortality is consistent with the 17 randomized placebo-controlled trials on which the FDA’s 2005 black box warning was based. These trials showed a 1.6 to 1.7 times greater risk of death associated with neuroleptic use in older people with dementia. (Average follow-up period: 10 weeks).
It is also noteworthy that although high neuroleptic dose entailed a greater relative risk of kidney damage than a low dose (1.80 vs. 1.73), the difference was slight. In other words, even low doses were associated with acute kidney injury.
DISCUSSION
On August 19, Medscape ran an article on this study. The author was Deborah Brauser. The article recounts the main findings of the study. Ms. Brauser also apparently interviewed Amit Garg, MD, the principal investigator. Here are some quotes from the article:
“‘I was actually surprised that this evidence was so robust. We did the study to determine what the association could be, and we clearly saw it,’ said Dr. Garg.”
“Because of this, Dr. Garg said he was surprised that so many patients were still receiving these prescriptions.
‘We looked at almost 100,000 adults in the province of Ontario who received these medications. This shows how commonly they’re used in just a single province, never mind worldwide,’ he said.
The researchers write that current evidence calls for a careful reevaluation of the prescribing of these medications in older adults.”
Also quoted in the Medscape article is Dilip V. Jeste, MD. Dr. Jeste is an eminent psychiatrist. He is a past president of both the APA and the American Association for Geriatric Psychiatry. He was not associated with the present study, but in his interview with Ms. Brauser he referred to an earlier study that he and some colleagues published in the Journal of Clinical Psychiatry in January 2013.
The objectives of that study were:
“To compare longer-term safety and effectiveness of the 4 most commonly used atypical antipsychotics (aripiprazole, olanzapine, quetiapine, and risperidone) in 332 patients, aged > 40 years, having psychosis associated with schizophrenia, mood disorders, posttraumatic stress disorder, or dementia, diagnosed using DSM-IV-TR criteria.”
And the results:
“Overall results suggested a high discontinuation rate (median duration 26 weeks prior to discontinuation), lack of significant improvement in psychopathology, and high cumulative incidence of metabolic syndrome (36.5% in 1 year) and of serious (23.7%) and nonserious (50.8%) adverse events for all atypical antipsychotics in the study.”
And the conclusions:
“Employing a study design that closely mimicked clinical practice, we found a lack of effectiveness and a high incidence of side effects with 4 commonly prescribed atypical antipsychotics across diagnostic groups in patients over age 40, with relatively few differences among the drugs. Caution in the use of these drugs is warranted in middle-aged and older patients.”
I found the last sentence quoted above puzzling. Given the findings, so clearly stated earlier, why not advocate cease and desist, rather than caution?
And in the Medscape interview, Dr. Jeste spoke very favorably of the current study:
“‘I think this is a really good study…'”
“‘It had a large sample size, and it was interesting how they selected people. They chose only those who were starting on the atypicals, which is appropriate. They also followed them for 90 days and did their best to have a similar control group.'”
But, again, puzzling:
“‘The bottom line is not that people should stop using these medications in older people. However, they should be more cautious, especially in patients with low blood pressure or evidence of kidney injury in the past.'”
There is this enormous reluctance among psychiatrists, even those who clearly have begun to see the light, to take a clear, unambiguous stand against harmful interventions. So often, they settle for the old face-saving caveat – “use caution”. But how can one use caution in prescribing a drug for an age group in which it has been shown to lack effectiveness and has a very high incidence of serious adverse effects? Surely the cautious approach would be not to use these drugs at all, especially since it’s virtually impossible to predict which individuals will suffer AKI or other adverse outcomes, including death.
Even Dr. Garg, principal investigator in the present study, backed off a cease and desist recommendation.
“The current available evidence calls for a careful reevaluation of prescribing atypical antipsychotic drugs in older adults, especially for the unapproved indication of managing behavioral symptoms of dementia…The drugs should be used only after other approaches have been exhausted; when prescribed, patients must be warned about potential adverse effects. Proactive clinical monitoring shortly after initiation seems reasonable (for example, serum creatinine and blood pressure measurement, and if readily available, a bladder scan to detect urinary retention). When patients present with AKI, atypical antipsychotic drugs should be considered a potential cause and be promptly discontinued if feasible.”
And in his interview with Ms. Brauser:
“‘We wanted to raise awareness around this issue and recommend that physicians should be cautious around the use of these medications in the elderly,’…”
And
“‘However, if a patient is on this medication and the physician is monitoring it very carefully, we don’t want to be alarmists. But it’s healthy to have these types of conversations.'”
ALSO INCIDENTALLY
The incidence of chronic kidney disease (CKD) is increasing among people 65 and over.
Here’s a graph from the National Kidney and Urological Diseases Information Clearinghouse:
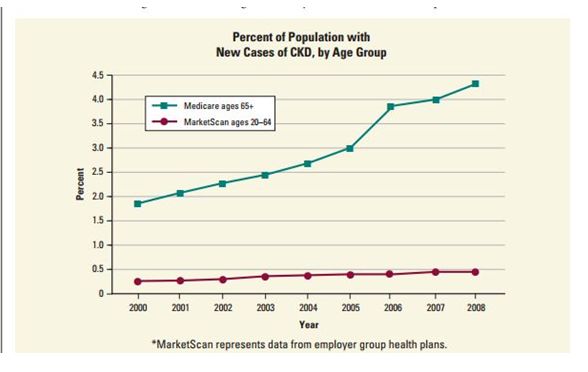 I’m not suggesting that all of this increase is due to neuroleptics. However, AKI often progresses to chronic kidney disease, and given the widespread prescribing of these products, it is reasonable to infer that the neuroleptics are a significant contributor.
I’m not suggesting that all of this increase is due to neuroleptics. However, AKI often progresses to chronic kidney disease, and given the widespread prescribing of these products, it is reasonable to infer that the neuroleptics are a significant contributor.
Here’s a quote from a 2009 editorial of the Clinical Journal of the American Society of Nephrology, The Nexus of Acute Kidney Injury, Chronic Kidney Disease, and World Kidney Day 2009, authors Mark Okusa, MD, et al.
“Acute kidney injury (AKI) is a devastating disease that affects patients throughout the world and is associated with high morbidity and mortality. It has been traditionally thought that patients who do survive recover renal function; however, recent population-based evidence strongly suggests that this may not be the case in many instances. New data suggest that a strikingly large percentage of patients who have AKI require permanent renal replacement therapy or do not fully recover renal function, and that this population has an important and growing impact on the global epidemiology of chronic kidney disease (CKD) and end-stage renal disease (ESRD).”
AND A FINAL NOTE
In 2009, Gwen Olsen published her book Confessions of an Rx Drug Pusher. In one of the chapters, she described how she systematically, and successfully, targeted nursing homes as markets for neuroleptic drugs.
There is a very poignant passage in which she describes, with considerable remorse and misgivings, how a particular resident – an elderly lady, exuberant and at times feisty – was reduced to a docile, inactive shell of her former self through a neuroleptic prescription.
Ultimately it’s a business. And it won’t stop until the cost of doing that business exceeds the profits. The findings of Dr. Garg and Dr. Jeste are helpful, of course They provide clear and unassailable evidence for what we already know: that in elderly populations, these drugs are being used as chemical straitjackets to “manage” agitation and aggression, and are killing people in the process.
But it is unlikely that polite calls for “caution” will stem the tide.