INTRODUCTION
I recently came across an article titled Diagnosisgate: Conflict of Interest at the Top of the Psychiatric Apparatus, by Paula Caplan, PhD. The article was published in Aporia, the University of Ottawa nursing journal, in January 2015. Aporia is “a peer-reviewed, bilingual, and open access journal dedicated to scholarly debates in nursing and the health sciences.”
Dr, Caplan is a clinical and research psychologist, and an Associate at Harvard’s DuBois Institute. She worked as a consultant to the DSM-IV task force in the 1980’s, but resigned from this position after two years. Here’s a quote from her February 2014 post on Mad in America The Great “Crazy” Cover-up: Harm Results from Rewriting the History of DSM:
“In the late 1980s, I was a consultant to two committees appointed by DSM-IV Task Force head Allen Frances to decide what DSM-IV should contain. I resigned from those committees after two years because I was appalled by the way I saw that good scientific research was often being ignored, distorted, or lied about and the way that junk science was being used as though it were of high quality . . . if that suited the aims of those in charge. I also resigned because I was increasingly learning that giving someone a psychiatric label was extremely unlikely to reduce their suffering but carried serious risks of harm, and when I had reported these concerns and examples of harm to those at the top, they had ignored or even publicly misrepresented the facts.”
Dr. Caplan has also written a brief synopsis of the Diagnosisgate article here.
PSYCHIATRISTS FOR HIRE
The central theme of Dr. Caplan’s 2015 article is that in 1995, Allen Frances, MD, and two other psychiatrists (John Docherty and David Kahn) received grants of about $515,000 from Johnson & Johnson to write “Schizophrenia Practice Guidelines” which specifically promoted Risperdal (a Johnson & Johnson product) as the first line of treatment for schizophrenia. The guidelines were called the “Tri-University Guidelines” in recognition of the fact that Dr. Frances worked at Duke, Dr. Docherty at Cornell; and Dr. Kahn at Columbia. Subsequently, the three psychiatrists formed Expert Knowledge Systems, and from that platform, actively assisted Johnson & Johnson in the implementation and marketing of the guidelines. For these latter services, J & J paid EKS a further $427,659.
The role of the three psychiatrists came to light because in 2004, the State of Texas filed a lawsuit against Janssen Pharmaceutica, a subsidiary of Johnson & Johnson, alleging that company officials “targeted Texas Medicaid with their sophisticated and fraudulent marketing scheme” (here) to ensure that Risperdal was included in the state’s preferred drug list.
During these proceedings, an expert witness report was presented to the court by David Rothman, PhD, professor of Social Medicine at Columbia University College of Physicians and Surgeons. The report is titled simply “Expert Witness Report” and is dated October 15, 2010. The report, which can be found here, runs to 86 pages, and is meticulously detailed.
Here are some quotes from Dr. Caplan’s article:
“Allen Frances, arguably the world’s most powerful psychiatrist, spearheaded a massive, million-dollar project using psychiatric diagnosis to propel sales of a potent and dangerous drug by pharmaceutical giant Johnson & Johnson (J & J). Frances began the initiative in 1995, but his involvement has been little known, despite a court document written in 2010 that revealed what its author [David Rothman, PhD], an ethics specialist, called serious deception and corruption in that project.”
“According to the court document, Frances led the J & J enterprise that involved distortion of scientific evidence, conflicts of interest, and other illegal and unethical practices.”
“Rothman reported that, in 1995, the very year after DSM-IV appeared, Johnson & Johnson had paid more than half a million dollars (USD) to Frances and two of his psychiatrist colleagues to create an official-seeming document as the basis for promotion of one of their drugs. The following year, the drug company paid them almost another half million dollars to continue and expand the marketing campaign.”
“According to the Rothman report, Frances and his colleagues wrote guidelines that were designed specifically to persuade physicians to prescribe J & J’s drug Risperdal as the first line of treatment for schizophrenia.”
Here are some quotes from David Rothman’s report:
“In 1993, GTFH Public Relations echoed what State and Federal Associates [another PR company] had recommended the year before. It, too, emphasized the need [for J & J]to cultivate state officials along with members of the psychiatric community…GTFH also emphasized that J&J should be convening Expert Task Force Meetings: ‘Formulate position and draft guidelines for consensus…Use: ‘Personalized invitational campaign to maximize participation.’…Finally, it counseled J&J to ‘Form exclusive partnership with growing advocacy group,’ citing NAMI as one case in point. J&J should help establish chapters and co-sponsor educational programs on patient issues…”(pp 13-14) [Emphasis added]
Note that one of GTFH’s recommendations was to “…draft guidelines…”
“As one of its first activities, and in disregard of professional medical ethics and principles of conflict of interest, in 1995 J&J funded a project led by three psychiatrists at three medical centers (Duke, Cornell, and Columbia) to formulate Schizophrenia Practice Guidelines. From the start, the project subverted scientific integrity, appearing to be a purely scientific venture when it was at its core, a marketing venture for Risperdal. In fact, the guidelines produced by this project would become the basis for the TMAP [Texas Medication Algorithm Project] algorithms, giving a market edge to the J&J products in Texas.” (p 14)
The production of the practice guidelines involved polling a selected sample of expert psychiatrists, and collating their questionnaire responses.
“The guideline team [Drs. Frances, Docherty, and Kahn] promised wide distribution of its product, including publication in a journal supplement. The team was prepared to have J&J participate in its work, not keeping the company even at arms length. With a disregard for conflict of interest and scientific integrity, the group shared its drafts with J&J. On June 21, 1996, Frances wrote Lloyd [John Lloyd, J&J’s Director of Reimbursement Services]: ‘We are moving into the back stretch and thought you would be interested in seeing the latest draft of the guidelines project….Please make comments and suggestions.‘ (Italics added). So too, the group was eager to cooperate with J&J in marketing activities. Frances wrote without embarrassment or equivocation: ‘We also need to get more specific on the size and composition of the target audience and how to integrate the publication and conferences with other marketing efforts” (Italics added)…Indeed, from the start J&J had made it apparent to the team that this was a marketing venture. In a letter to Frances, Lloyd set forth what he called an ‘aggressive time line’ for the project, and added: ‘There are a number of other Treatment and Practice Guidelines for schizophrenia being developed or published during this same period that may well serve our marketing and implementation needs at a substantial lesser cost.’…” (p 15)
“Not only were Frances, Docherty and Kahn ready to violate standards of conflicts of interest in mixing guideline preparation with marketing for J&J, but also in publicizing the guidelines in coordination with J&J. The three men established Expert Knowledge Systems (EKS). The purpose of this organization was to use J&J money to market the guidelines and bring financial benefits to Frances, Docherty, and Kahn.” (p 15)
“EKS [i.e., Drs. Frances, Docherty, and Kahn] wrote to Janssen on July 3, 1996 that it was pleased to respond to its request to ‘develop an information solution that will facilitate the implementation of expert guidelines.’…It assured the company: ‘We are also committed to helping Janssen succeed in its effort to increase its market share and visibility in the payor, provider, and consumer communities.’ Now that the ‘first phase’ was completed, with the guidelines created, ‘EKS is now ready to move forward in a strategic partnership with Janssen.’…The strategy will allow Janssen to ‘Influence state governments and providers…. Build brand loyalty and commitment with large groups of key providers around the country.’…EKS also promised ‘rapid implementations,’ with particular attention to having an impact on Texas decision making.’It is our intent to work with the State of Texas immediately in implementing this product in a select number of CMHC’s with the assistance of A. John Rush, MD.’…Again EKS emphasized: ‘It is essential for Janssen to distinguish Risperidone [Risperdal] from other competitors in a timely and creditable way.’…In its Summary of the document, EKS wrote: ‘Your investment in the development of state of the art practice guidelines for schizophrenia is already beginning to pay off in terms of positive exposure in the Texas Implementation project.’…” (p 15-16) [Emphasis added]
Back to Paula Caplan’s article:
“On August 30, 2012, Texas Attorney General Abbott issued a press release to announce that Texas and 36 other states had together reached a settlement in which Janssen was to pay the states a total of $181 million because of its ‘unlawful and deceptive marketing.’ Here there appears another mystery: Interestingly, nowhere in either the filing or the press release did the names of Frances, Docherty, or Kahn appear, although their deceptive guidelines were the foundation for the enterprise, nor did they include the names of the other psychiatrists whom Janssen had hired to carry out the deceptive acts. Furthermore, they did not include information about harm done to the individuals who had been prescribed Risperdal.”
“Papers impelled by J & J were published in scholarly journals and, as Rothman reports, ghost-written by individuals selected by J & J, with high-profile names affixed as first authors after the articles had been written. These papers helped promote use of Risperdal to treat not only Schizophrenia but also Childhood Onset Schizophrenia, Schizo-affective Disorder, Bipolar Disorder in Children and Adults, Mania, Autism, Pervasive Developmental Disorder other than Autism, Conduct Disorder, Oppositional Defiant Disorder, Psychosis, Aggression Agitation, Dementia, below average IQ, and disruptive behavior. Subsequent to the production and marketing of the Tri-University Guidelines came the FDA approval of Risperdal to treat adults and then children diagnosed with Bipolar Disorder, and finally children diagnosed with Autism.”
And another quote from David Rothman:
“J&J turned the guidelines into a powerful marketing tool. The slides presented at a CNS National Sales Meeting in March 1997, instructed employees to use the guidelines to convince its ‘Primary customers: P & T members [Pharmacy and Therapeutics committees], Formulary Decision Makers and Psychopharmacologists’ – those who made purchasing and reimbursement decisions – that they should use the guidelines to justify making Risperdal the drug of choice.…J&J also wanted the guidelines to promote the product’s use among ‘Secondary Customers,’ namely ‘Physicians who are not convinced of RISPERDAL’s 1st line status.’ So although the front piece for the guidelines described them as ‘suggestions for clinical practice,’ from J&J’s perspective, they provide ‘credibility; Reinforces RISPERDAL’s 1st line status; Differentiates RISPERDAL from convention[al] APS [antipsychotics] and other atypical APS.’ To make certain the customers got the message, the ‘Full Supplement [of the guidelines publication] should be left behind.’ J&J also funded CME offerings to publicize the guidelines, including a ‘Free ½ Day Seminars, Earn Up to 8 Hours of CE/CME.’ The panel of experts included Frances, Docherty, and Kahn, and also John Rush (who would play a key role in TMAP). http://web.archive.org/web/19961106071503/www.ibh.com/expert1.htm” (p 17)
. . . . . . . . . . . . . . . .
What’s particularly noteworthy in all of this is that since about 2010, Dr. Frances has been critiquing the obviously expansionist agenda of DSM-5, and the corruptive role of pharma in disease-mongering, and in the increasing over-use of psychiatric drugs.
In this context, he presents himself as the defender of moderation and scientific integrity, but, to the best of my knowledge, he has never publicly acknowledged his marketing role with J & J in the creation of the Tri-University Guidelines.
ALLEN FRANCES REPLIES
On March 5, 2015, Dr. Frances did respond to Paula Caplan’s “Diagnosisgate” article. Here are some quotes from this response, which appeared on the Huffington Post blog. The quotes are interspersed with my comments.
“…in her usual dramatic and distorted way, Dr. Caplan feels she can score points and gain public attention by exposing a supposed, creatively named, ‘Diagnosisgate.'”
It is my general perception that when people respond to criticism with this kind of personal attack, they have something to hide.
. . . . . . . . . . . . . . . .
“Dr. Caplan, as always, is careless with facts, quick with misinterpretations, and filled with wild accusations. I will first debunk what is simple nonsense in her claims and then discuss the issues that do have a factual basis.”
“It is nonsense to state that my participation in guideline development was in any way a conflict of interest with DSM IV or affected in any way its preparation. The guideline project occurred several years after DSM IV was already in print. The term ‘Diagnosisgate’ is no more than Dr Caplan’s misleading attempt to attract an audience and has no connection to reality.”
There is no suggestion in Dr. Caplan’s article that Dr. Frances’s participation in the guideline development was a conflict of interest with DSM-IV. In fact, Dr. Caplan notes that J & J’s first payment to Drs. Frances, Docherty, and Kahn occurred the year after DSM-IV was published. What’s stressed in both Paula Caplan’s and David Rothman’s reports is the fact that Dr. Frances and his two co-founders of EKS actively collaborated with Johnson & Johnson in the marketing of Risperdal, and that the guidelines that they created were clearly designed for this purpose.
. . . . . . . . . . . . . . . .
“It is nonsense to imply that I made a great deal of money from DSM IV sales, which Dr. Caplan states totalled $100 million.”
There is no reference, or even implication, in Dr. Caplan’s article, that Dr. Frances made a great deal of money from DSM-IV sales. Dr. Caplan mentions the fact that sales of the manual “earned more then $100 million”, but there is no suggestion that Dr. Frances shared in these profits. Again, what’s stressed in both Paula Caplan’s and David Rothman’s articles is that Dr. Frances and his colleagues made about $900,000 from J & J for producing and marketing the Tri-University Guidelines.
. . . . . . . . . . . . . . . .
“It is nonsense for Dr. Caplan to claim there was ‘data distortion’ in either DSM IV or in the guidelines. Both efforts were the result of completely transparent and forthright processes. Both efforts had very clear and published methodological rules of the road that were conscientiously followed every step of the way.”
The phrase “data distortion” occurs in a sub-heading in Dr. Caplan’s synopsis article, but the phrase does not occur in her main article in Aporia. So I’m not sure exactly what she had in mind. But the notion that following one’s own “clear and published methodological rules of the road” guarantees validity and lack of bias is a little naïve. All that Drs. Frances, Docherty, and Kahn would have to do to skew the results is, firstly, select questionnaire recipients whom they knew favored risperidone, and secondly, word the questions in a way that would tend to elicit the kind of responses that would promote risperidone. In the published guideline document, the authors state that questionnaire participants were selected from several sources:
“…recent research publications and funded grants, the DSM-IV advisers for psychotic disorders, the Task Force for the American Psychiatric Association’s Practice Guideline for the Treatment of Patients with Schizophrenia, and those who have worked on other schizophrenia guidelines.” (p 2)
From this – obviously very large group – Dr. Frances and his partners selected 99 psychiatrists, 87 of whom responded to the questionnaire. I can find no information as to how the 99 psychiatrists were selected.
. . . . . . . . . . . . . . . .
“She enjoys being the center of controversy and will always do her best to stir a tempest in a thimble.”
Ah! That explains everything.
. . . . . . . . . . . . . . . .
But then, Dr. Frances acknowledges some retrospective misgivings:
“But in retrospect, there are two things about the project I much regret. Firstly, it was very unwise to do guidelines with drug industry funding. Even though they were fairly done, accurately reported, and contained built in methodological protections against industry-favorable bias, the industry sponsorship by itself created an understandable appearance of possible bias that might reduce faith in the sound advice and useful method contained in them. It was an error in judgment on my part that I apologize for. I have learned from my mistake and hope others do as well.”
So, there was absolutely nothing wrong with the guidelines, but the acceptance of pharma money may have created an appearance of bias. And although no such bias existed, Dr. Frances is apologizing for creating this appearance. But remember the EKS commitment quoted earlier: “We are also committed to helping Janssen succeed in its effort to increase its market share and visibility in the payor, provider, and consumer communities.” This is a clear statement of bias. It’s not an appearance of bias; it’s not possible bias. It is out and out, unmitigated bias. They express commitment, not to some improvement in client outcomes or welfare, but to increasing Janssen’s market share. Drs. Frances, Docherty, and Kahn were hired to market Risperdal. They were well paid, and they delivered what their employer expected of them.
And incidentally, despite his misgivings, there’s no indication that Dr. Frances has refunded his share of the $900,000 from J & J. “May one be pardon’d and retain the offence?” (Hamlet, Act 3, Scene 3)
. . . . . . . . . . . . . . . .
“Secondly, I did not at the time anticipate, nor did the experts, that the atypical antipsychotics would be so frequent a cause of obesity and of the serious complications that follow from it. The considerable risks involved in using these new medications, and ways of avoiding these, were then unknown and not covered in the guideline.”
So, he assumed without evidence that the drug was safe unless proven dangerous, when in fact, good practice would be the opposite: assume that the drug is dangerous, until it’s proven safe! But, in any event, it wasn’t Dr. Frances’s fault. After all, who could have known?
PUBLICATION OF THE GUIDLELINES
In 1996, the EKS’s Treatment of Schizophrenia guidelines were published as a 58-page supplement in The Journal of Clinical Psychiatry. It’s an interesting document, and it certainly does promote the use of Risperdal (risperidone). But of even more interest is this statement in the preface to the supplement . It was written by Alan Gelenberg, MD, Editor in Chief of the journal. Dr. Gelenberg begins the Preface with some words of praise for the guidelines, but also advocates caution with regards to pharma-funded projects:
“…in conditions such as bipolar disorder and schizophrenia, where the primary treatments are medications, industry is a looming presence. Pharmaceutical companies devote enormous sums to academic departments and individual faculty members who consult, conduct research, and teach under the auspices of the company. These then are the experts who create consensus guidelines. While few of us sell our opinions to the highest bidder, fewer still are immune from financial influence.” [Emphasis added]
So Dr. Gelenberg could see these issues very clearly in 1996, when the guidelines were published; but Dr. Frances, despite his mea culpa in the Huffington Post last March, still hasn’t grasped the issue. In that document, from which I quoted earlier, Dr. Frances contends that the guidelines “…contained built in methodological protections against industry-favorable bias…”. But as Dr. Gelenberg so clearly points out, the expert consultants on whose opinions the guidelines were based were already subject to industry influence by the very fact of their status within the psychiatric community. So, in fact, industry-favorable bias was actually built in.
Page 2 of the supplement lists the 87 expert psychiatrists on whose questionnaire responses the guidelines were based. The list is in alphabetical order, and I checked the first fifteen names for links to pharma. Two of the fifteen are deceased. Of the remaining thirteen, nine have disclosed that they have received payments from pharmaceutical companies, and eight of these have received payments from Janssen Pharmaceutica/J & J.
I have no way of checking if these financial links were present in 1995/96 when the guidelines were produced, but the extent of these individuals’ involvement with pharma today suggest that Dr. Gelenberg’s concerns were probably well founded.
. . . . . . . . . . . . . . . .
On the supplement’s sub-cover there is a brief acknowledgement of Janssen’s funding:
“This project was supported by an unrestricted educational grant from Janssen Pharmaceutica.”
The term “unrestricted” has a very specific meaning in this context. It means that the recipients of the grant are not required to produce any particular result. Essentially there is an expectation that both grantor and recipient will take steps to keep one another at arms’ length. The term “unrestricted” is, in a sense, a warranty to the reader that the document in question is free from funder bias.
In the light of the material quoted above, it strikes me that the description “unrestricted” in this case was at best misleading, and possibly a blatant deception.
A CHALLENGE TO DR. FRANCES
If Dr. Frances really wants to put this matter to rest, he needs to answer these questions publicly and unambiguously:
- Are the allegations against him and his EKS partners that are set out in detail in the David Rothman report accurate?
- Are the quotations in that report that are attributed to Dr. Frances accurate?
If the answer to both of these questions is No, then I suggest that Dr. Frances start devoting his time and energy to addressing these matters, and clearing his name, because the allegations are very serious.
But if the answer to one or both of these questions is Yes, then I respectfully suggest that Dr. Frances exit the stage with whatever dignity he can muster, and resume his well-earned retirement.
AND INCIDENTALLY
Mickey Nardo, MD, has also posted David Rothman’s report on his website, 1 Boring Old Man. Dr. Nardo has also written a post on this topic. The post is titled detestable.
. . . . . . . . . . . . . . . .
I have quoted from David Rothman’s report in this post, but I’ve confined my attention to material concerning Dr. Frances and EKS. In fact, the report covers a lot more ground, and gives a great deal of detail on the pharma-psychiatry corruption that has marred the landscape in this field for so long. It’s well worth reading.
For instance, here’s an insightful little gem from page 21:
“Shon [Steven Shon MD, Medical Director of the Texas Department of Mental Health and Mental Retardation] was also considered a pivotal figure by another J&J employee, Percy Coard…After thanking his colleagues for attending a Shon presentation, he listed all the reasons why J&J wanted a ‘strategic alliance’ with him. As Coard explained, Shon was a KOL [key opinion leader], influential in the public sector, where ’85 Percent of all anti-psychotic dollars come from;’ he has influenced and supported the use of new drugs in TMAP [Texas Medication Algorithm Project], and a proactive approach to him ‘to support/partner with his current and future projects in the public sector arena will continue to position Janssen as a true partner in public mental health initiatives.'”
Such a sense of civic responsibility!
. . . . . . . . . . . . . . . .
Robert Whitaker discusses EKS and the Tri-University Guidelines in his latest book, Psychiatry Under the Influence, p 149-150.
FINALLY
And for anyone who has any doubts concerning the effectiveness of pharma-psychiatry’s marketing machine, here’s a graph produced by the Agency for Healthcare Research and Quality (AHRQ), a division of the US Department of Health and Human Services.
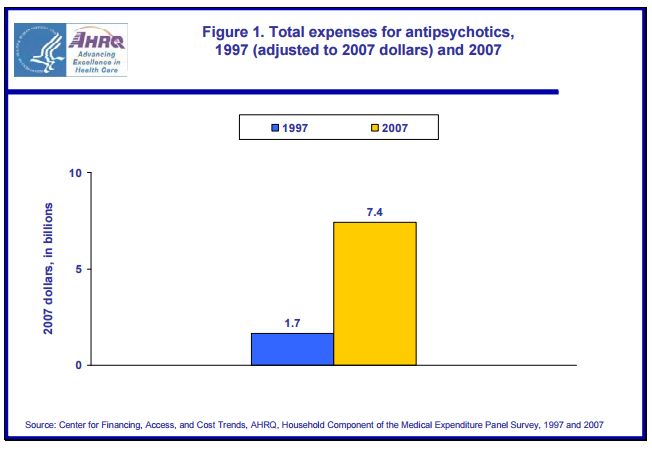
So between 1997 and 2007, total expenses for neuroleptic drugs in the US went from $1.7 billion (corrected to 2007 value) to $7.4 billion. This is an increase of $5.7 billion over and above any increase due to inflation.
The cost of these extra sales in terms of reduced life expectancy and quality of life is psychiatry’s legacy to humanity.